Учебное пособие Санкт-Петербург 2009 удк 802. 0
| Вид материала | Учебное пособие |
- Учебное пособие Санкт-Петербург 2011 удк 621. 38. 049. 77(075) Поляков, 643.33kb.
- Учебное пособие Санкт- петербург 2010 удк 778. 5 Нестерова Е. И, Кулаков А. К., Луговой, 708kb.
- Учебное пособие Санкт-Петербург 2005 удк 662. 61. 9: 621. 892: 663. 63 Ббк г214(я7), 546.15kb.
- Учебное пособие Санкт-Петербург 2007 удк алексеева С. Ф., Большаков В. И. Информационные, 1372.56kb.
- Учебное пособие санкт Петербург 2010 удк 001. 8 Ббк, 1217.72kb.
- Учебное пособие Санкт-Петербург 2011 удк 1(075., 3433.28kb.
- Учебное пособие санкт-петербург 2005 удк 339. 9 (075. 80) Ббк, 703.64kb.
- Учебное пособие Санкт-Петербург 2000 удк 681, 344.56kb.
- Учебное пособие для студентов среднего профессионального образования специальности, 2227.42kb.
- Учебное пособие для студентов среднего профессионального образования Санкт-Петербург, 2212.78kb.
3. Nanotubes
Much of excitement in nanochemistry has been in the manufacture and characterization of nanotubes. These are essentially made from just carbon atoms arranged into hexagonal rings with pentagonal rings to close the structure at the ends. Either single or multi-walled tubes are shown to be formed from concentric nanotubes They appear to have huge tensile strength .
Bundels of nanotubes may be shown to be in the region of 50 times stronger than steel. Reseach is currently under way to use carbon nanotubes to create transistors and other electronic devices much smaller than can be created using silicon chips. By inserting silver halides into singlewalled nanotubes and the decomposing them to silver, metallic nanowire of pure silver with diameter 2,0X10
 m have been made, which are reported to be the thinnest electrical wires in existence.
m have been made, which are reported to be the thinnest electrical wires in existence.Nanotechnology has the potential to solve many problems, such as increasing food production, preventing, monitoring and curing diseases, and improving information and communication technology, although most of these benefits still are likely to lie somewhere far in the future ( 1222 печатных знака).
4. Cross-linking in polymers
When polymers are able to form giant three-dimensional structures, as for example in phenol-methanal plastics, it makes the polymer extremely strong, because a large amount of energy is required to break it down. It also makes them insoluble and generally unreactive.
Another example of this is Kevlar, the material which is known to be used in production of lightweight bullet-proof vests, composites for motor-cycle helmets and armour.
Kevlar is a polyamide made by condensing 1,4,-diaminobenzene( para-phenylenediamine) with benzene-1,4-dicarbonyl chloride( terephthaloyl dichloride). A strong three-dimensional structure in this polymer is believed to be formed due to hydrogen bonding between the long rigid chains.
Polymers can be modified by various means during their manufacture. For example, air can be blown into polyurethane to make polyurethane foams for use as cushions and thermal insulation. The fibers of polyesters can be blended with other manufactured or natural fibers to make clothes such as skirts that are more comfortable, and which can hold dyes fast ( 1175 печатных знаков).
5. Silicon and photovoltaic cells
Metals are known to conduct electricity well when voltage is applied , because they contain delocalized electrons. Silicon is a semiconductor. A crystal of silicon is shown to contain a lattice of silicon atoms bonded to each other by share of paired electrons. These electrons are in fixed positions , so silicon is a poor conductor under normal conditions However, the energy required to excite an electron and free it from its bonding position is equivalent to the energy of light with a wavelength of 1.1x10
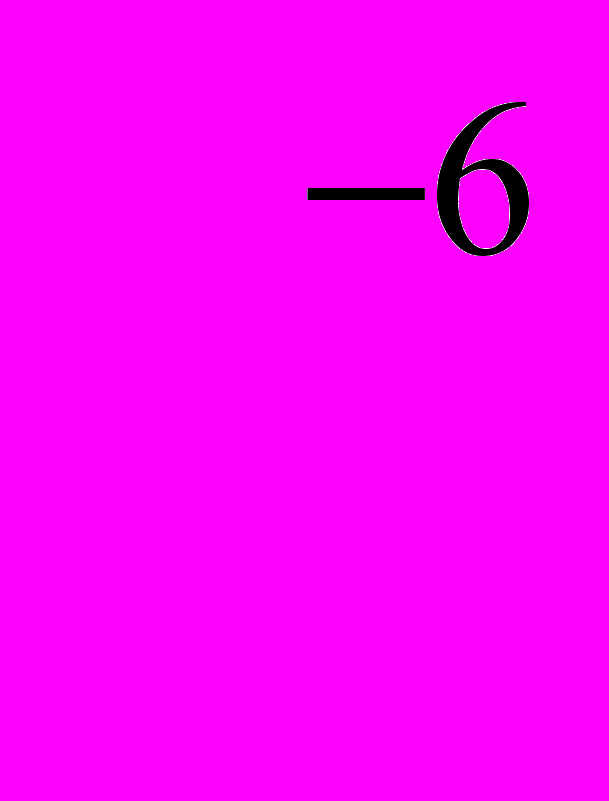 m. So sunlight is able to excite an electron in silicon. The electron is then free to move through the crystal lattice when voltage is applied, making silicon an electrical conductor. In practice the process does not appear to be very efficient and the cost of purifying the silicon is high. To improve the efficiency of photoelectric effect doping need to be used. Adding very small amount of other elements one can produce n-type semiconductors as well as p- type semiconductors. The junction between the two different types of semiconductors allows the current to flow in one direction only. Such a junction is known to be a rectifier and can be used to convert alternating current into direct (1342 печатных знаков).
m. So sunlight is able to excite an electron in silicon. The electron is then free to move through the crystal lattice when voltage is applied, making silicon an electrical conductor. In practice the process does not appear to be very efficient and the cost of purifying the silicon is high. To improve the efficiency of photoelectric effect doping need to be used. Adding very small amount of other elements one can produce n-type semiconductors as well as p- type semiconductors. The junction between the two different types of semiconductors allows the current to flow in one direction only. Such a junction is known to be a rectifier and can be used to convert alternating current into direct (1342 печатных знаков).6. Research, development and testing of new pharmaceutical products
The research and development of new drugs is a long and costly process, but the financial rewards to pharmaceutical companies can be high. To test a new product it is necessary to isolate it from an existing species or synthesize it chemically and then subject to thorough laboratory and clinical pharmacological studies to demonstrate its effectiveness. It is tested on animals to determine the lethal dose required to kill 50 % of animal population. The effective dose required to bring about noticeable effect in 50 % of population is also obtained so that the safe dose to administer can be determined. Then the drug is likely to be used in initial clinical trial on humans. This is usually on consenting volunteers as well as patients, half of whom are given the drug and half of whom are given a similar-looking placebo. The volunteers do not know whether they have been taking the drug or placebo .This initial trial is closely monitored to establish the drug’s safety and possible side effects
(1237 печатных знаков).
7. Ethanol
Because alcohol is stated to impair judgment and slow down the body’s reflexes, most countries have strict legal limits for permissible amount of alcohol in the blood for motorists, airline pilots and people operating machinery. There are several ways to determine the amount of alcohol in the body. In past motorists might be asked to blow into a breathalyzer. This involved acidified potassium dichromate(VI) crystals turning green as they were reduced by the ethanol to chromium (III) ions, or use of a fuel cell, where the ethanol was oxidized to produce electricity. These methods are not sufficiently accurate to be used in court. At police station a blood sample may be taken and sent to a forensic science laboratory. Modern intoximeters can now be used to measure accurately the amount of alcohol in the breath. They are known to be based on the principle that the carbon –to-hydrogen bonds in the ethanol absorb infrared radiation of a particular wavelength. Ethanol can interact with and considerably enhance the effect of other drugs because it depresses the central nervous system . With aspirin it increases the risk of stomach bleeding, because the alcohol helps to transport aspirin through the protective mucus lining of the stomach wall (1484 печатных знаков).
8. Historical development of penicillin
The use of moulds to prevent infections has existed in folklore for a long time. A Costa Rican, Clodomiro Picardo Twight working at the Pasteur Institute in Paris was the first to record the action of the fungal genus Penicillium sp. on the growth of bacteria and the work was published in 1927. However, the discovery of penicillin is known to be attributed to the Scotsman Alexander Fleming.
Fleming, a bacteriologist, was working with cultures of Staphylococcus aureus, a bacterium that causes boils and other types of infection. In 1928 hе left an open petri dish containing one of the cultures in the laboratory while he went away on holiday. Upon his return he noticed a mould to have developed and inhibited growth of the bacterium. He deduced the mould produced a compound ( which he called penicillin ) that inhibited the growth of bacteria. The discovery of penicillin is often quoted as an example of serendipity-the accidental discovery of something useful while looking for something else. In the 1950s the structure of penicillin was determined, and this enabled chemists to synthesize different types of penicillin and other antibiotics ( antibacterials originating from moulds) (1222 печатных знаков).
9. Dissolved oxygen in water
When matter decomposes aerobically in water it uses up the dissolved oxygen. The biological ( or biochemical) oxygen demand (BOD) is a measure of the dissolved oxygen ( ppm) often measured over a set time period of 5 days. Water that has a high BOD without the means of replenishing oxygen will rapidly fail to sustain aquatic life. A fast –flowing river is likely to recover its purity, because the water becomes oxygetared through the mechanical action of its flow. Lakes have relatively little flow, and reoxygenation appears to be much slower or does not happen at all. Pure water has a BOD of less than 1ppm, water with a BOD above 5ppm is known to be regarded as polluted (744 печатных знаков).
10. Waste water treatment
Waste water treated at sewage works contains floating matter, suspended matter, colloid matter, dissolved ions and a range of micro-organisms. The aim of the treatment is to remove this additional material and recycle the fresh water. Primary treatment is supposed to remove about 60% of solid material and about a third of the oxygen-demanding wastes. The incoming sewage is passed through coarse mechanical filters to remove large objects such as sticks, paper, and rags. It is then passed into a grit chamber, where sand and small objects settle. From there it passed into a sedimentation tank, where suspended solids settle out as sludge. A mixture of calcium hydroxide and aluminium sulfate is added to aid this process.
The two chemicals combine to form aluminium hydroxide, which precipitates, carrying with it suspended dirt particles ─ a process which is known to be called flocculation. Secondary treatment is likely to remove up to 90 % of oxygen demanding wastes. The principle is to degrade the waste aerobically using oxygen and bacteria. Tertiary treatment is aimed to remove heavy metal ions, phosphates and nitrates. Nitrates are difficult to remove by chemical means as all common nitrates are soluble, so precipitation cannot be used.
Anaerobic denitrifying bacteria can reduce them to nitrogen, or the water can be passed through algal ponds, where the algae utilize the nitrate as a nutrient (1542 печатных знаков ).
11. Antioxidants
Antioxidants are assumed to delay the onset of oxidation, or slow down the rate at which it occurs. Although some occur naturally , they are also added to extend the shelf life of food. Naturally occurring antioxidants are known to include vitamin C, vitamin E ,b- carotene, selenium. All of them are found to contain a phenolic group ,and many contain a carbon atom bonded directly to three methyl groups , which is known as a tertiary butyl group. Both the phenolic group and the tertiary butyl group are considered to be free radical scavengers. They are shown to react with and remove the free radicals involved in the oxidation of food and thus prolong the shelf life. They are believed to enhance the health effects of other foods, and boost overall health and resilience. However, synthetic antioxidants which are classed as food additives need to be regulated by policies and legislation to ensure their safe use in food (1122 печатных знаков).
12. Nanotechnology
Nanotechnology is a field of applied science and technology covering a broad range of topics. The main unifying theme is the control of matter on a scale smaller than 1 micrometer, normally between 1-100 nanometers , as well as the fabrication of devices on this same length scale. It is known to be a highly multidisciplinary field, drawing from fields such as colloid science, device physics, and supramolecular chemistry.
The term “nanotechnology” was defined by Tokyo Science University Professor Norio Taniguchi in a 1974 paper as follows:” Nanotechnology “ mainly consists of processing, separation, consolidation, and deformation of materials by one atom or by one molecule”.
Nanotechnology and nanoscience are considered to have started in the early 1980s with major developments : the birth of cluster science and the invention of the scanning tunneling microscope(STM). These developments led to the discovery of fullerenes in 1986 and nanotubes a few years later.
A unique aspect of nanotechnology is the vastly increased ratio of surface area to volume present in nanoscale materials, which opens new possibilities in surface - based science, such as catalysis.
Scientists showed nanomaterials to posses different properties compared to what they exhibit on a macro scale. Due to that fact unique applications for nanomaterials can be found (1524 печ. зн.).
12 Тесты по теме инфинитив и инфинитивные конструкции (ESP).
Переведите следующие предложения на русский язык.
Тест №1
1. This substance is found to be useful.
2. The radius of our orbit is believed to be increasing very slowly.
3. They proved to have provided all for the experiment.
4. There appear to be two distinct effects in this case.
5. The lithium nucleus is too small for so many collisions (столкновение-) to occur.
6. Alpha-rays were shown to be identical, по matter from what radioactive element they are emitted.
7. In liquids and solids the movement of molecules must be supposed to be more restricted.
8. Only а limited number of reactions are known to be influenced by light
9. Such reaction was not observed to happen.
10. Many materials which may appear to be amorphous are really crystalline in structure.
11. Hydrogen does not appear to combine with clorine with appreciab velocity in the dark.
12. Uranium was the first element which was found to possess the property of radioactivity.
Тест №2
1. A substance is known to possess these properties is called an acid.
2. This was the cubic equation which was believed in ancient Egypt to be insoluble.
3. Secondary radiation may be expected to rise when the primary radiations are observed.
4. Electrons can be made to travel at very high speed.
5. The speed of the particles is a bit too swift (быстрый) for us to study in detail.
6. We consider the hotness or coldness of any body to depend on the quantity of heat possessed by the body.
7. Properties of substances are their characteristic quality which may be used to describe them.
8. Pressure causes ice melt.
9. This apparatus enables accurate measurements to be carried out with ease.
10. The first organic compound isolated by chemists was wine.
11. The effect is too small to be detected.
12. In order for two molecules to react they must be in contact.
Тест №3
1. For a reaction to take place, an A molecule must first meet а В molecule.
2 А small crystal of ice added, to the supercooled water is sufficient to cause the арреаrance of icе.
3. It is possible to compress this substance,
4 Не heard the bell ring.
5. Strong acids Arrenius assumed to be highly ionized.
6. This theory was the first concept of matter to be based upon experimental evidence.
7. He seems to know this rule well.
8. I happened to be out when he called.
9. Z was taken to be equal 9.
10. This substance has been isolated in а free form and has been state to fluoresce in the dark.
11. А star gradually cools down and can be said to undergo an evolutiоnаy change.
12. Аbove - 100˚ the oxide (F2O2) decomposed slowly to give what was at first thought to be а new oxide FO.
Тест №4
1. He is always the first to come.
2. The substance to be analyzed should be pure.
3. There will be six independent elements to be determined.
4. Attractive forces may make molecules со11ide.
5. А catalyst аllows a greater quantity of the products of а reaction to be manufactured.
6. I was not able to write my test. It proved to be too difficult.
7 The guests are likely to arrive soon.
8. The results are expected to agree with theoretical predictions.
9, Such reactions was not observed to happen.
10. Water proved to be а compound.
11. During the Dark Ages (средние века) people believed the Earth to be flat.
12. А11 students are supposed to know Newton’s laws of ' mechanics.
Тест№5
1. А11 substances are known to have some ability to transmit electrons.
2 Some liquids prove to be good conductors.
3. This metal is likely to be brittle (хрупкий) at the temperature mentioned.
4. Salt water appears to conduct electricity well.
5. We know electric current to flow in metal parts.
6. Everybody considers electronic devices to play a great role in industrial control.
7. They have seеn the device begin to operate,
8. There are many problems to be solved in order to understand these phenomene.
9. The lecture to be followed by an experiment is to take place at our institute.
10. Crookes was the first to recognize the cathode rays as negatively charged particlaes.
11. The mechanical systems are often too simple to be of any practical interest.
Тест №6
1. То observe is the primary rule of any experiment.
2. We use lines of force as did Faraday, so as to picture the electric field more clearly.
3. It is impossible for people to work at very high temperatures at 200˚ for instance.
4. The time required for the equilibrium to be established varies greatly.
5. What makes a moving body stop?
6. Elasticity is the property of matter that enables it to keep its original form.
7. Many years ago scientists believed an electric current to be а from of energy.
8. We know electric current to be surrounded by a magnetic field.
9. A voltmeter is an instrument to be used for measuring the potenti al difference between any two points in a circuit.
10. Today scientists in order to describe mass and length use the metric system of units.
11 Care should be taken for the conditions of work to be changed and made normal.
12. He chanced to observe an unusual effect.
Тест №7
1. Falling on а special kind of cell, light can cause an electric current to flow.
2. What causes а body to move? Isaac Newton was the first to answer this question.
3. А copper atom has 29 electrons; at least one of them can be easily made to leave the atom.
4. Lomonosov proved heat to be а form of energy.
5. All molecules are supposed to be in motion.
6. We know the strength of а current to depend upon the resistance of the circuit.
7. When the currents to be detected and measured are very small, one should use а galvonometer.
8. The lecture to be followed by an experiment to take place at our Institute.
9. The precipitate was observed, to dissolve slowly.
10. Silicon is only known to form either four or six bonds.
11. Аir was considered by the encients to be element.
12. For the tests to be comparable the tests should be made exactly in the same way.
Тест №8
1. The copper wire that connects two plates in an electric cell forms a very easy path for the electrons to move.
2. This law is generally taken to аррlу to all gases аnd their mixtures.
3. Uranium was the first element which was found to possess the proрerty of radioactivity.
4. This substance is known to dissolve in hot water.
5. This substance is to be found in nature.
6. Light is to be considered as some kind of wave motion of electromagnetic origin.
7. Ions have been found by numerous experiments to move as easily through а jell (гель) as through the liquid solution.
8. Their method was to add radon to the substance to be examined.
9. They watched the temperature rise gradually.
10. Assume the total preassure to be equal to 10.
11. Other gases were found 1о behave like air,
12. The eclipse (затмение) of the sun was predicated to occur in 1460.
Тест №9
1. The metal to be found in this ore is of great value.
2. Аttractive forces may make molecules collide.
3. Chlorides can be made by methods to be described later.
4. Which method is to be chosen in this case?
5. Unfortunately the thermal conductivity is very hard to determine.
6. In this experiment we are to compare the relative weight of two substances.
7. The question is not simple to answer.
8 This is the first factor to be taken into consideration.
9. The time taken for equilibrium conditions to be set is small.
10. It is not possible for a liquid to be compressed nearly as much as a gas.
11. They found radon to be times as heavy as hydrogen.
12. As present time, 96 substances are known which chemists consider to be elements.
Тест №10
1. We know to have been a difficult problem to solve.
2. Lasers proved to be of great help in medicine and industry.
3. To explain this simple fact is not so very easy.
4. It takes the rays of the sun 8 minutes to get to the Earth.
5. Phosphorus is too active element to be found free in nature.
6. Elements combine to produce a compound.
7. One way to obtaining hydrogen is to pass electric current thru water.
8. The next step will be produced a diagram of the system.
9. We observed the evaporation of water, a phenomenon to be more fully described later.
10. The report to be followed by a concert will take place at 8 o’clock.
11. The first liqueified gas to be used as a refrigerant on a large scale was ammonia.
12. Here is one more important point for the speaker to explain.
Тест №11
1. Deformation appeared to have no measurable effect on conductivity
2. Let us take the volume of this body to equal v.
3. The temperature was too low for the substance to decompose.
4. Polonium was the first of the radioactive elements to be isolated by the chemists.
5. These methods are to be described in the next chapter.
6, There are some more figures to be referred to latex.
7. The atom may eject (изгонять) another particle to become в different atom.
8, To give а true picture of the surrounding matter is the task of natural science.
9. The waves are too short to affect the eye.
10, Lead (свинец) will dissolve in sodium solution to give green solutions.
11. The problem has been to solidify the substance under investigation.
12. Experiments have proved the pressure of а gas at fixed temperature to depend on its concentration.
